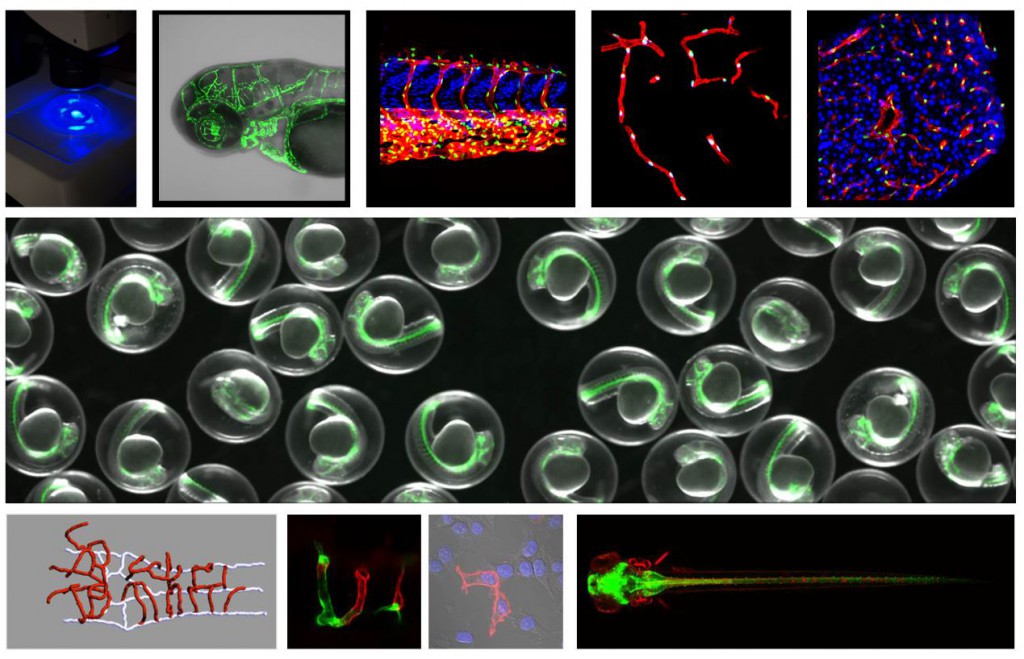
We explore the processes of brain angiogenesis and blood-brain barrier formation by combining genetic approaches and single-cell resolution imaging in zebrafish embryos.
Elaborating on the findings permitted by this potent genetic discovery model, we seek to reach a mechanistic understanding of the processes that couple vascular and neural development through complementary approaches in cell biology, mouse genetics as well as cell-free biochemical and biophysical technologies.
Proper brain function relies on intricate communications between the neural and vascular systems. Endothelial cells lining the brain blood vessels elaborate a convoluted tubular network, keeping the high energy-consuming neurons within a few microns distance from blood-borne nutrients and dissolved gases. The fluctuating composition of the blood plasma being incompatible with reliable synaptic communications, brain endothelial cells are in addition endowed with a set of molecular, cellular, and metabolic adaptations that stringently orchestrate the molecular and cellular transit at the interface between the brain and the circulatory system. Collectively called the blood-brain barrier (BBB), they result from complex and dynamic communications within the neurovascular unit and ensure brain homeostasis. Through its neuroprotective function, the BBB represents a stubborn obstacle for CNS drug delivery, impeding an overwhelming majority of neuroactive molecules from reaching effective concentrations in target brain regions. Conversely, BBB breakdown contributes to a large set of neurological disorders, including stroke and neurodegenerative diseases. Therefore, we need to better understand neurovascular signaling in health and disease.
Brain angiogenesis
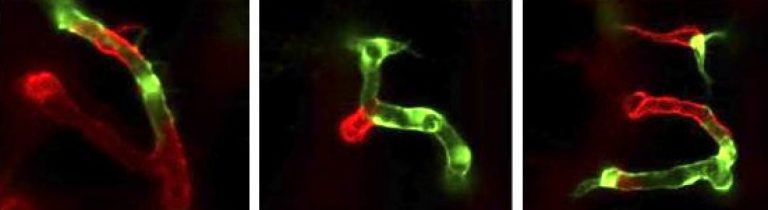
During embryogenesis, endothelial cells acquire organ-specific characteristics to adapt to the requirements of the perfused organs and tissues. The brain vascular microenvironment serves as a paradigm for extreme blood vessel specialization (blood-brain barrier, BBB), where endothelial cell adaptation appears to be under the direct control of a distinct angiogenic program. Recent evidence suggests that brain angiogenesis and the expression of some BBB markers are temporally and functionally coupled. Both processes appear not to be genetically ‘hard-wired’ but instead induced by neuroepithelium-derived cues. Only in recent years have some of the molecular pathways that initiate brain angiogenesis and govern blood-brain barrier formation been revealed. Identifying these organ-specific angiogenic regulators and ordering them into pathways may constitute a unique opportunity to modulate brain vessel formation in brain disease. We explore these pathways in zebrafish embryos through a combination of targeted mutagenesis (CRISPR/Cas9, TALEN), genetic mosaics, and single-cell resolution real-time imaging. Our laboratory has more particularly been investigating an unusual membrane receptor complex of CNS endothelial cells, composed of Gpr124 and Reck, that has emerged as a key regulator of neurovascular biology and a prime therapeutic target for brain disorders with neurovascular implications.
Blood-brain barrier formation
A key challenge in the treatment of brain diseases is overcoming the difficulty of delivering diagnostic or therapeutic agents to specific regions of the brain. Chief among these obstacles is the neuroprotective Blood-Brain Barrier (BBB), both a physical and metabolic barrier to most macromolecules and small compounds. We explore BBB formation and function in the genetically tractable zebrafish model to improve our understanding of the basic molecular and cellular mechanisms that regulate brain microvasculature permeability in vertebrates, with the overarching goal to develop innovative strategies for drug or gene targeting to the injured or diseased brain.